Sample information |
|
| Picture |



|
|---|---|
| Location | |
| Collection date | 09/26/2025 |
| Captive / Cultivated? | Wild-caught |
| Group | Berkshire Community College |
| Observations | It was outside near the corner of the building in moist dirt near leaves and rocks. |
| Putative identification | Arthropoda Malacostraca Isopoda Armadillidae |
Methods |
|
| Extraction kit | Monarch DNA extraction (NEB) |
| DNA extraction location | Abdomen |
| Single or Duplex PCR | Duplex Reaction |
| Gel electrophoresis system | Edvotek Gel Electrophoresis |
| Buffer | 1X TAE |
| DNA stain | SYBR Safe |
| Gel images |

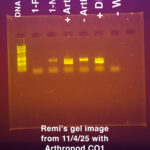
|
| Protocol notes | Our first PCR reaction was set up on 10/14/25 and was a duplex reaction because we used both the Arthropod CO1 and Wolbachia 16S primes together. We used an annealing temperature of 49 degrees celsius. We used this Taq polymerase: New England Biolabs OneTaq Hot Start Quick-Load 2X Master Mix with Standard Buffer (#M0488S).
We used a DNA extraction protocol based on the insect adaptation of New England Biolabs Monarch Spin gDNA Extraction kit. (Product #T3010). The specimen was incubated for 30 minutes in a hot water bath at 56 degrees C.
Our second PCR reaction was set up on 10/28, we used the same Taq polymerase as the first PCR reaction and it was a duplex reaction that used Arthropod C01 and Wolbachia 16S primers. We used an annealing temperature of 49 degrees celsius. Our second gel image was taken on 11/4/25 and was run at 125 volts for 25 minutes. We used the New England Biolabs 1 kb Plus DNA Ladder for safe stains (product #N0559S) |
Results |
|
| Wolbachia presence | Unknown |
| Confidence level | High |
| Explanation of confidence level | My arthropod tested positive for Wolbachia, but it did not have a band for arthropod DNA. Therefore, I am not confident in the results. I ran another duplex PCR, and my results showed nothing for Wolbachia. There was also no band showing any arthropod DNA, so I am confident that something went wrong in the beginning, maybe the DNA extraction. |
| Wolbachia 16S sequence | |
| Arthropod COI sequence |
|
| Summary | |
 European Wasp
European Wasp Small Honey Ant
Small Honey Ant 7A
7A Millipede 7F
Millipede 7F