Sample information |
|
| Picture |



|
|---|---|
| Location | |
| Collection date | 09/26/2025 |
| Captive / Cultivated? | Wild-caught |
| Group | Berkshire Community College |
| Observations |
|
| Putative identification | Arthropoda Insecta Lepidoptera Noctuidae Papaipema Papaipema inquaesita |
Methods |
|
| Extraction kit | Monarch DNA extraction (NEB) |
| DNA extraction location | Abdomen |
| Single or Duplex PCR | Duplex Reaction |
| Gel electrophoresis system | Edvotek Gel Electrophoresis |
| Buffer | 1X TAE |
| DNA stain | SYBR Safe |
| Gel images |

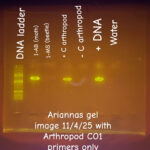
|
| Protocol notes | We used a DNA extraction protocol based on the insect adaptation of New England Biolabs’ Monarch Spin gDNA Extraction kit (Product # T3010) The specimen was incubated for 30 minutes in a hot water bath at 56 degrees C. Our second Gel image was taken on 11/4/25 and was run at 125 volts for 25 minutes used this DNA ladder: New Englands Biolabs 1 kb Plus DNA Ladder for Safe Stains (product #N0559S)
On our second PCR reaction was set up on 10/28/25, used the same Taq polymerase as the first PCR reaction, and: was a single reaction that used the Arthropod primers used an annealing temperature of 49 degrees Celsius. |
Results |
|
| Wolbachia presence | No |
| Confidence level | High |
| Explanation of confidence level | I am confident that my experimental results were valid. We followed protocol exactly and were very cautious about cross contamination. The bands look as they should- clear, bright, and the accurate size.
I am still confident that my results were valid. We used Arthropod CO1 primers for our single PCR and Wolbachia bands were clearly not present the first time we did Gel Electrophoresis and followed protocol exactly. |
| Wolbachia 16S sequence | |
| Arthropod COI sequence | Download AB1
CAAATTTATAATACTATTGTTACAGCTCATGCTTTTATTATAATTTTTTTTATAGTTATACCCATTATAATTGGAGGATTTGGAAATTGACTTGTACCTTTAATATTAGGAGCTCCTGATATAGCATTTCCACGAATAAATAATATAAGTTTTTGATTACTTCCACCCTCATTAACTTTATTAATCTCAAGAAGAATTGTAGAAAATGGAGCTGGAACAGGATGAACAGTGTACCCCCCACTTTCATCTAATATTGCTCATGGAGGAAGTTCTGTAGATTTAGCAATTTTTTCCCTTCATTTAGCTGGTATTTCCTCCATCTTAGGAGCTATTAATTTTATTACCACAATTATTAATATACGACTAAATAATTTATCTTTTGATCAAATACCTTTATTTATTTGAGCTGTAGGTATTACTGCATTTTTATTATTACTATCATTACCTGTTTTAGCAGGAGCTATCACAATATTATTAACAGATCGAAATTTAAATACATCATTTTTTGATCCAGCAGGAGGAGGAGACCCAATTTTATACCAACATTTATTTTGAT
BLAST at The Wolbachia Project BLAST at NCBI
|
| Summary | The Papaipema inquaesita was found to be negative for Wolbachia. |
 European Wasp
European Wasp Small Honey Ant
Small Honey Ant 7A
7A Millipede 7F
Millipede 7F