Sample information |
|
| Picture |
|
|---|---|
| Location | |
| Collection date | 09/06/2025 |
| Captive / Cultivated? | Wild-caught |
| Group | Edmund Burke School |
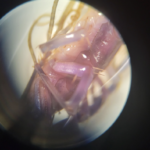
| Observations | Caught in an open garage in a grassy, woody, suburban area. Outside the garage has many types of different vegetation, along with many concrete or asphalt paths. Caught late summer/early starts of fall Temperature: High of 91F; Low of 68 Terrestrial Habitat Color: Before putting it in the alcohol, its color was a tanish brown, with some areas being a darker brown or black. After being put into the alcohol, the color in a number of areas changed to a blue and purple color scheme. Wings: No wings Legs: Many legs, more than 8, that go around most of the arthropod’s body. The forelegs are just behind the head. The hind legs Antennae: 2 long and slender antennae, looking similar to a piece of thread. Body: The body seems to have a more oval shape. However, it does seem to be segmented somewhat into two sections: the head and the body. The head is a rounded shape, containing compounded eyes, long antennas, and forcipules. The mouth is also small, with the forcipules begin behind the mouth. It doesn’t have a distinct thorax or abdomen.
|
| Putative identification | Arthropoda Chilopoda Scutigeromorpha Scutigeridae Scutigera Scutigera coleoptrata |
Methods |
|
| Extraction kit | DNeasy (Qiagen) |
| DNA extraction location | Abdomen |
| Single or Duplex PCR | Single Reaction |
| Gel electrophoresis system | MiniOne |
| Buffer | TBE |
| DNA stain | GelGreen |
| Gel images |
|
| Protocol notes | DNA Extraction: For this process, only the abdomen was used. During this, this sample did have some type of bubbles before we incubated the sample. Additionally, this sample solution was a purplish blue color, which differed from the tanish color of the others. No mistakes were made for this sample that we are aware of.
PCR: For the PCR process, we did a CO1 one and a Wolbachia one. In the overall PCR process, there was a mistake where the DNA of 2 of the 6 samples was not added for the PCR one. However, this particular sample wasn’t one of those, leading to no known mistakes for A-5.
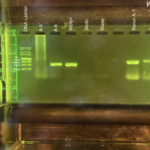
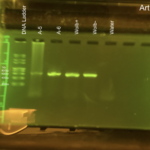
Gel electrophoresis, Arthropod CO1: We used two different machines for the CO1 gel electrophoresis machines. For machine 1: Lane 1 – DNA Ladder Lane 2 – Arthropod A-1 Lane 3 – Arthropod A-2 Lane 4 – Arthropod A-3 Lane 5 – Arthropod A-4 Lane 6 – Wolbachia (+) control Lane 7 – Wolbachia (-) control Lane 8 – Water Lane 9 – Blank
For machine 2: Lane 1 – DNA Ladder Lane 2 – Arthropod A-5 Lane 3 – Arthropod A-6 Lane 4 – Wolbachia (+) control Lane 5 – Wolbachia (-) control Lane 6 – Water Lane 7 – Blank Lane 8 – Blank Lane 9 – Blank
All of the controls worked, with the Wolbachia (+) control and Wolbachia (-) control having the CO1 band and the water control having no band, meaning no contamination was made. Meaning that any mistakes made, in other words no bands showing up for arthropod sample, was because of an error in either the PCR process or loading the gel electrophoresis machine. This sample did have a band show up that was readable, however, it did appear smudged. However, we continue with the results from the gel since the band was at the correct location, which is 708bp.
Gel electrophoresis, Wolbachia: We used two different machines for the Wolbachia gel electrophoresis machines. For machine 1: Lane 1 – DNA Ladder Lane 2 – Arthropod A-1 Lane 3 – Arthropod A-2 Lane 4 – Arthropod A-3 Lane 5 – Arthropod A-4 Lane 6 – Wolbachia + control Lane 7 – Wolbachia – control Lane 8 – Water Lane 9 – Blank
For machine 2: Lane 1 – DNA Ladder Lane 2 – Arthropod A-5 Lane 3 – Arthropod A-6 Lane 4 – Wolbachia + control Lane 5 – Wolbachia – control Lane 6 – Water Lane 7 – Blank Lane 8 – Rerun Arthropod A-1 Lane 9 – Rerun Arthropod A-2
The reruns at the end of the machine 2 gel electrophoresis were because no CO1 band showed up on that gel for those samples due to the DNA being forgotten in the PCR process. All of the controls worked, with the Wolbachia (+) control having a Wolbachia band at 438bp and the Wolbachia (-) control and the water control having no band, meaning no contamination was made. Meaning that any mistakes made, in other words no bands showing up for arthropod sample, was because of an error in either the PCR process or loading the gel electrophoresis machine. This sample did not have a Wolbachia band that showed up, however like in the CO1 gel electrophoresis, it did appear smudged. However, even though the gel was smudge, we concluded that the sample was negative after looking at it more and a professional option. |
Results |
|
| Wolbachia presence | No |
| Confidence level | High |
| Explanation of confidence level | My confidence level is what it is because everything seemed to go according to plan with the process. With this, all of the controls worked properly and there were no known mistakes made in the process of this sample. The only part that might have changed this confidence level was the smudging of both gel electrophoresis of this sample. However, I’m not really worried about it since the bands that appeared were readable, in the right location, and all the controls were correct. |
| Wolbachia 16S sequence | |
| Arthropod COI sequence |
|
| Summary | The Scutigera coleoptrata was found to be negative for Wolbachia. |
 European Wasp
European Wasp Small Honey Ant
Small Honey Ant 7A
7A Millipede 7F
Millipede 7F