Sample information |
|
| Picture |









|
|---|---|
| Location | |
| Collection date | 09/23/2025 |
| Captive / Cultivated? | Wild-caught |
| Group | Berkshire Community College |
| Observations | The arthropod was found on top of a mostly still pool of water at the edge of a brook. It was skidding across the water with others of the same species. It was collected just after dark on a day that had light rain throughout the afternoon. The temperature at the time of collection was about 17.8 degrees C (64 degrees F).
*Note that the arthropod was identified as Chironomidae sp. by NCBI BLAST using its DNA sequence (from the CO1 gene), but that identification was not considered accurate based on the observations of the collected arthropod. Body structure and behaviors before collection did not line up with those expected from Chironomidae sp.. Based on observations the arthropod is deemed to be a water strider of an undetermined species (Gerris sp.).* |
| Putative identification | Arthropoda Insecta Hemiptera Gerridae Gerris |
Methods |
|
| Extraction kit | Monarch DNA extraction (NEB) |
| DNA extraction location | Abdomen |
| Single or Duplex PCR | Duplex Reaction |
| Gel electrophoresis system | Edvotek Gel Electrophoresis |
| Buffer | 1X TAE |
| DNA stain | SYBR Safe |
| Gel images |
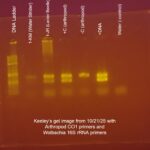
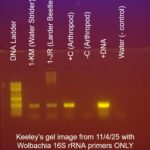

|
| Protocol notes |
|
Results |
|
| Wolbachia presence | Yes |
| Confidence level | Medium |
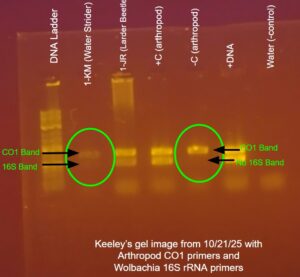
| Explanation of confidence level | I am moderately confident in the presence of Wolbachia in my arthropod because both the first and second PCR showed a band for the Wolbachia 16S gene and both reactions had expected outcomes from the controls. Although it was extremely faint, it looked as though my first gel electrophoresis had picked up a DNA strand of about 440 base pairs (I compared my arthropod’s lane (2) with the lane containing the -Arthropod Control (5) to identify the presence of a Wolbachia band). Since the band for the DNA of my arthropod was also faint, it can be assumed that the DNA was fully extracted out of my arthropod, making Wolbachia DNA much harder to identify.
With a single PCR using Wolbachia 16S primers only, a more conclusive result was determined. Because it was run at an annealing temperature of 55 degrees Celsius it had a much higher sensitivity for the Wolbachia 16S gene. Our second gel electrophoresis showed a band at about 440 base pairs in my arthropod sample, signifying the presence of Wolbachia in my arthropod.
Even through my results are clear, it is very possible that they show a false positive. Water striders are predatory arthropods and with the misidentification from NCBI BLAST it is likely that DNA from another arthropod that the water strider consumed was extracted. If this is the case, it cannot be determined whether the water strider was infected or if it consumed a Wolbachia infected arthropod before collection. |
| Wolbachia 16S sequence | Download AB1
GGGGTGGCGGTTTCCCGGTGTTGCATGGCTGTCGTCAGCTCGTGTCGTGAGATGTTGGGTTAAGTCCCGCAACGAGCGCAACCCTCATCCTTAGTTACCATCAGGTAATGCTGGGGACTTTAAGGAAACTGCCAGTGATAAACTGGAGGAAGGTGGGGATGATGTCAAGTCATCATGGCCCTTATGGAGTGGGCTACACACGTGCTACAATGGTGGCTACAATGGGCTGCAAAGTCGCGAGGCTAAGCTAATCCCTTAAAAGCCATCTCAGTTCGGATTGTACTCTGCAACTCGAGTGCATGAAGTTGGAATCGCTAGTAATCGTGGATCAGCACGCCACGGTGAATACGTTCTCGGGTCTTGTACACACTGCCCGTCACGCCATGGGAATTGGTTTCACTCGAAGCTA
BLAST at The Wolbachia Project BLAST at NCBI
|
| Arthropod COI sequence | Download AB1
TTATTTTTTTGGAGCTTGATCTGGAATAGTAGGAACTTCCCTAAGTATACTTATTCGAGCAGAATTAGGCCATCCTGGCACATTTATTGGAGATGACCAAATTTATAATGTTATTGTAACAGCTCATGCTTTCATTATAATTTTTTTTATAGTTATACCTATTTTAATTGGAGGTTTTGGTAATTGACTTTTACCTTTAATACTAGGAGCCCCTGATATAGCATTCCCACGAATAAATAATATAAGTTTTTGATTATTACCCCCTTCTCTTTCTTTACTTCTTTCTAAAAGAATTGTAAAAAATGGTGCAGGAACTGGATGAACAGTTTATCCACCTTTATCTTCCAAAATTGCACATAGAGGGGCTTCAGTCAATTTAGCTATCTTTTCTCTTCATCTAGCCGGAATTTCTTCTATTCTTGGGTCTGTAAATTTTATTACAACAGCTATCAATATACAATCAAATGGAATCTCATTAAATCGAATACCATTATTTGTATGATCTGAAATTATTACAACAATCTTACTACTTATTTCTCTTCCTGTATTAGCCGGAGCCATTACTATACTTTTAACAAACCGAAATTTAAATACATCTTTCTTTGACCCTGCAGGAGGAGGAAACCCAATTTTATACCAACATCTATTTTGATTTTTTGGCCCCCCGGAAGTATTAAA
BLAST at The Wolbachia Project BLAST at NCBI
|
| Summary | The Gerris was found to be postive for Wolbachia. |
 European Wasp
European Wasp Small Honey Ant
Small Honey Ant 7A
7A Millipede 7F
Millipede 7F