Sample information |
|
| Picture |






|
|---|---|
| Location | |
| Collection date | 09/30/2025 |
| Captive / Cultivated? | Wild-caught |
| Group | Berkshire Community College |
| Observations | Collected around 06:30 pm outside on the east side of the collector’s home. The arthropod was observed walking quickly along a brick walkway. |
| Putative identification | Arthropoda Insecta Hymenoptera Formicidae Lasius Lasius neoniger |
Methods |
|
| Extraction kit | Monarch DNA extraction (NEB) |
| DNA extraction location | Whole arthropod |
| Single or Duplex PCR | Duplex Reaction |
| Gel electrophoresis system | Edvotek Gel Electrophoresis |
| Buffer | 1X TAE |
| DNA stain | SYBR Safe |
| Gel images |
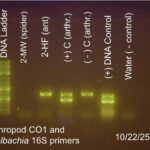

|
| Protocol notes |
Our first PCR was set up on 10/15/25 and:
Our first gel image was taken on 10/22/25 and
Our second PCR was set up on 10/29/25, used the same Taq polymerase as the first PCR reaction, and:
Our second Gel image was taken on 11/5/25 and:
|
Results |
|
| Wolbachia presence | No |
| Confidence level | High |
| Explanation of confidence level |
For results of gel electrophoresis run on 10/22/25 using the DNA ladder in lane 1 as a band size reference in bp (base pairs), I was able to determine sample 2-HF (Laius ant) in lane 3 had one band around 700bp, showing the presence of the CO1 gene, which confirms successful arthropod DNA extraction. No other bands were present, indicating no Wolbachia 16S was detected in sample 2-HF. The controls in lanes 3 through 7 performed as expected with the (+) arthropod control showing a band at 700bp for CO1 and a band around 400bp indicating the presence of 16S Wolbachia. The (-) arthropod control only showed one band at 700bp for the C01 gene. The (+) DNA control also showed 2 bands indicating presence of the CO1 gene and Wolbachia 16S. The final control in lane 7 was the sterile, nuclease-free water which showed no bands and a faint primer dimer. I have a high confidence level in the results based on the success of the controls and the clarity of the results.
For results of gel electrophoresis run on 11/5/25 using the DNA ladder in lane 1 as a band size reference in bp (base pairs), we see again that sample 2-HF (Laius ant) in lane 3 had one band around 700bp, showing the presence of the CO1 gene, which confirms successful arthropod DNA extraction. The controls in lanes 3 through 7 performed as expected with the (+) arthropod and (-) arthropod controls and the (+) DNA control all showed a band at 700bp for CO1 as expected. The final control in lane 7 was the sterile, nuclease-free water which showed no bands as it should. I have a high confidence level in the results based on the success of the controls and the clarity of the results. |
| Wolbachia 16S sequence | |
| Arthropod COI sequence | Download FASTA
Download AB1
GAGCTGGTATAATCGGCTCATCTATAAGAATAATTATTCGTCTAGAATTAGGATCATCTAATTCATTGATTAATAATGATCAAATTTATAATTCTATAGTTACAAGACATGCATTCATTATAATTTTCTTTATAGTTATGCCTTTCATAATTGGAGGATTTGGAAATTTCCTTGTACCTTTAATATTAGGCTCACCTGATATAGCTTATCCACGTATAAACAATATGAGATTTTGACTTTTACCCCCCTCAATTTCTCTTCTTATTTTAAGAAATTTTATTAATGATGGTGTTGGGACAGGATGAACTGTTTATCCTCCTTTAGCTTCAAATATTTTCCATAATGGACCTTCAGTTGATTTAACTATTTTTTCCCTTCATATTGCTGGTATATCTTCAATTTTAGGAGCTATTAATTTTATTTCAACTATCTTAAATATACATCACAAAAATTTTTCTATTGATAAAATCCCTTTACTTGTATGATCAATTTTAATTACTGCAATTTTACTTCTCTTATCCTTACCCGTTCTTGCAGGAGCTATTACTATACTTTTAACTGACCGTAATCTTAACACTTCATTTTTTGATCCATCAGGTGGCGGAGATCCTATTTTATATCAACATCTTTTTTGA
BLAST at The Wolbachia Project BLAST at NCBI
|
| Summary | The Lasius neoniger was found to be negative for Wolbachia. |

 European Wasp
European Wasp Small Honey Ant
Small Honey Ant 7A
7A Millipede 7F
Millipede 7F