Sample information |
|
| Picture |

|
|---|---|
| Location | |
| Collection date | 09/26/2025 |
| Captive / Cultivated? | Wild-caught |
| Group | Berkshire Community College |
| Observations | Hairy, hides in shadows, small |
| Putative identification | Arthropoda Arachnida Araneae Salticidae |
Methods |
|
| Extraction kit | Monarch DNA extraction (NEB) |
| DNA extraction location | Whole arthropod |
| Single or Duplex PCR | Duplex Reaction |
| Gel electrophoresis system | Edvotek Gel Electrophoresis |
| Buffer | 1X TAE |
| DNA stain | SYBR Safe |
| Gel images |
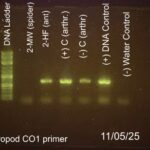
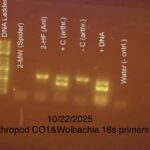
|
| Protocol notes | 1. we used a DNA extraction protocol based on the insect adaption of New England Biolabs’ Monarch Spin gDNA Extraction kit 2. the specimen was incubated for 30 minutes in a hot water bath at 56 degrees C 3. Details that differed from the written protocol were in step 16 when liquids were uneven LAB 7 1. Our first PCR reaction was set up on 10/15/2025
LAB 8
Lab 9 10/28 – Our second PCR reaction was set up on 10/29/2025, used the same Taq polymerase as the fist PCR reaction and:
Lab 10
|
Results |
|
| Wolbachia presence | Unknown |
| Confidence level | Low |
| Explanation of confidence level | all 4 controls match my expectations, the 2nd lane had an error with the DNA process as it was not fully extracted causing no DNA to show up in the lane. lab 10
|
| Wolbachia 16S sequence | |
| Arthropod COI sequence |
|
| Summary | |
 European Wasp
European Wasp Small Honey Ant
Small Honey Ant 7A
7A Millipede 7F
Millipede 7F