Sample information |
|
| Picture |

|
|---|---|
| Location | |
| Collection date | 02/18/2025 |
| Captive / Cultivated? | Captive / Cultivated |
| Group | Berkshire Community College |
| Observations | |
| Putative identification | Arthropoda Insecta Orthoptera Gryllidae Acheta Acheta domesticus |
Methods |
|
| Extraction kit | Monarch DNA extraction (NEB) |
| DNA extraction location | Rear half |
| Single or Duplex PCR | Duplex Reaction |
| Gel electrophoresis system | Edvotek Gel Electrophoresis |
| Buffer | TAE |
| DNA stain | SYBR Safe |
| Gel images |


|
| Protocol notes | For DNA the incubation time (1 minute) with the elution buffer could have been surpassed or underestimated. No timer was set, we based our time off of how long other students let their DNA sit for. Other than that we followed the directions/protocol the whole way through. Taq polymerase used were the New England Biolabs OneTaq Hot Start. For the PCR with the arthropod Primers the annealing temperature was 49 C. The Taq polymerase that were used was the New England Bioloabs OneTaq Hot Start Quick-Load 2x Mm w/ Standard Buffer (product #M0488s). The DNA Ladder that was used was the New England Biolabs 1kb Plus DNA Ladder for Safe Stain (product #N0559S). This was our second attempt to get a clear stain from the arthropods, the date the duplex PCR was set up was March 26, 2025, the annealing temperature was 49 degrees Celsius. |
Results |
|
| Wolbachia presence | No |
| Confidence level | High |
| Explanation of confidence level | My confidence level was high that my arthropod did not have Wolbachia. During the Duplex PCR the DNA bands were very clear but the Wolbachia bands were not there causing the conclusion that my arthropod was not infected. All of the controls were as expected. |
| Wolbachia 16S sequence | |
| Arthropod COI sequence | Download AB1
NNNNNNNNNNNNNNNGTTNTTCNTTTGNCTGTCTCNNGCTCTTTGTCANANANNNTTGGTTTAATTCCCCCAACNACCGC ANCCTTNTTCCTTATTTACCATCNGGTAANGCTGGGAACTTNAAGGAAACTGCCANTGATANCTGAANAANGGTGGGNNG ANTGTCNTNNCANCTGGCNNTTNNNGGATTGNGCTNCCNCNTGCTACAATGGTGGCTACAANGGGCTGCAAATTCGCAAG GCTTANCTAANNCTNNAAANCCATCTCATTTCGAANTGNACTCTGCCCTCNAGTGCATGAATTTGGAATCNCNAGAANNN TGGAATCACCACCCCACGNNAAATACTTTCTCGGGTCTGGTACACCCTGCCCGTCACCCCTGGGAAATTGTTNNNNNTTA ANNCNNNN
BLAST at The Wolbachia Project BLAST at NCBI
|
| Summary | The Acheta domesticus was found to be negative for Wolbachia. |
 Ant like creature – Draft
Ant like creature – Draft Myrmaplata plantaleoides (Unsure) – Draft
Myrmaplata plantaleoides (Unsure) – Draft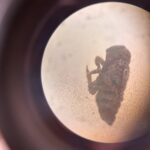 leaf hopper nymph
leaf hopper nymph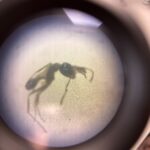 Pheidole species (Minor Worker)
Pheidole species (Minor Worker) JTR 1
JTR 1