Sample information |
|
| Picture |


|
|---|---|
| Location | |
| Collection date | 08/30/2020 |
| Captive / Cultivated? | Wild-caught |
| Group | Bordenstein Lab |
| Observations | I was walking through a wildflower park near Edwin Warner Park in Nashville, TN and this sweat bee landed on my arm. It was collected by quickly placing a collection vial over the bee and immediately capping with a lid. It was about 80F and very sunny. |
| Putative identification | Arthropoda Insecta Hymenoptera Halictidae Lasioglossum |
Methods |
|
| Extraction kit | DNeasy (Qiagen) |
| DNA extraction location | Whole arthropod |
| Single or Duplex PCR | Single Reaction |
| Gel electrophoresis system | MiniOne |
| Buffer | TBE |
| DNA stain | GelGreen |
| Gel images |
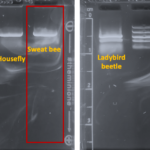
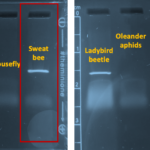
|
| Protocol notes | This sweat bee was stored in 70% ethanol for about 3 weeks prior to DNA extraction. DNA Extraction: The specimen was incubated in lysis buffer at 56C for 2 hours. Because there was cell debris, I did a 30 sec spin and transferred the supernatant to a fresh tube of 200ul ethanol. This was placed in the freezer overnight and DNA was purified the following day. Eluted DNA was immediately incubated at 65C for one hour prior to PCR. PCR: MiniOne Taq polymerase was used. Gel electrophoresis: The arthropod gel looks whispy; however, that went away as the gel ran longer. This could have been caused by not adding loading dye to samples. The results were very clear, though. I re-colored the gel images in PowerPoint. The MiniOne ladder contains 5 bands: 100, 300, 500, 1000, and 2000 bp. |
Results |
|
| Wolbachia presence | Yes |
| Confidence level | High |
| Explanation of confidence level | The DNA extraction was successful because the arthropod COI amplified. Both (+) and (-) controls worked. The Wolbachia 16S rRNA band was present. The Sanger sequence was very low quality due to non-specific binding. However, the best sequence (most resolved peaks) show BLASTn homology to the genus Lasioglossum. This genus represents sweat bees and is the largest of all bee genera. Therefore, it is likely a species of Lasioglossum. As with the arthropod DNA, the Wolbachia Sanger sequence was also too low-quality to analyze. While this could be due to a Wolbachia co-infection, the quality of the host DNA implies that it may likely be from the DNA extraction. |
| Wolbachia 16S sequence | Download AB1
|
| Arthropod COI sequence | Download AB1
|
| Summary | The Lasioglossum was found to be postive for Wolbachia. |
 European Wasp
European Wasp Small Honey Ant
Small Honey Ant 7A
7A Millipede 7F
Millipede 7F