Sample information |
||
| Picture |


|
|
|---|---|---|
| Location | ||
| Collection date | 09/09/2024 | |
| Captive / Cultivated? | Wild-caught | |
| Group | Owego Free Academy | |
| Observations | Formicidae colony found near the Owego creek under nearby rocks. Specimens were alive at the time of collection. |
|
| Putative identification | Arthropoda Insecta Hymenoptera Formicidae | |
Methods |
||
| Extraction kit | DNeasy (Qiagen) | |
| DNA extraction location | Abdomen | |
| Single or Duplex PCR | Duplex Reaction | |
| Gel electrophoresis system | MiniOne | |
| Buffer | TBE | |
| DNA stain | GelGreen | |
| Gel images |
|
|
| Protocol notes |
|
|
Results |
||
| Wolbachia presence | No | |
| Confidence level | Low | |
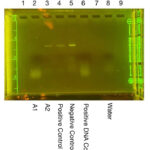
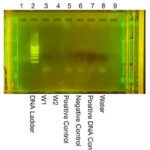
| Explanation of confidence level | Arthropod Gel Imaging Analysis(Figure 3): Lanes 1 and 9 were left intentionally empty in this gel, thus no DNA bands were magnified. Lane 2 (A1) was pipetted with a solution that should have had our first arthropod’s DNA within it, yet did not yield a band. This means that the Arthropod Barcoding Gene (CO1) was poorly prepared or improperly placed in the well; alternatively, it may have been a result of the low-level of solution left in the PCR tube. Lane 3 (A2) was representative of our second arthropod, and a clear band was visible, thus the arthropod DNA was successfully extracted. Lane 4 (Positive Control) developed a CO1 band, meaning the process used to extract and prepare the arthropod DNA was successful for this sample. Lane 5 (Negative Control) also developed a defined band, implying the intentional presence of a CO1 gene and the success of this control. Lane 6 (Positive DNA Control) had no presence of a CO1 band. This lane should have had a CO1 band, and the failure of this control is likely a result of degradation of the sample, PCR failure, or a lack of sample left in the PCR tube. Lane 8 (water) was a negative control, containing only water and no DNA. No band was present in this lane, meaning this control succeeded. Wolbachia Gel Imaging Analysis(Figure 4): Lanes 1 and 9 were left intentionally empty in this gel, thus no DNA bands were magnified. In the gel (excluding the DNA Ladder lane), the loading dye is visible, and beneath it are bands of what could be DNA, but it most likely primer dimers, which would mean that no DNA was magnified, in this trial(excluding the DNA Ladder). The following summary entertains the idea that these bands are DNA: Lane 2 (DNA Ladder) successfully displayed the DNA ladder, proving the success of the electrophoresis. In Lane 3 (W1), a very faint band was present, meaning that if no contamination occurred, Wolbachia was present in this arthropod. In Lane 4 (W2), no band was present, resulting from the arthropod not being infected, or from a lack of sample left in the PCR tube (much of the sample was compromised in the first trial). Lane 5 (Positive Control) did have a blurred band present, meaning that the control did work. Lane 6 (Negative Control) was the negative arthropod control and should not have yielded a band, yet did. This control failure is indicative of contamination, likely during the DNA extraction. Lane 7 (Positive DNA Control) did not yield a Wolbachia band as it was intended to, and this result, paired with the previous, implies a degradation of the sample or a result of indistinct labeling. Lane 8 (water) was meant to be a negative control and should not have yielded a band, yet it did. This could indicate contamination, likely during the PCR reaction. However, it is also highly possible that the poor labeling of the PCR tubes resulted in the water and Positive DNA controls being swapped, thus explaining the failure of both of these controls within this experiment. |
|
| Wolbachia 16S sequence |
None
BLAST at The Wolbachia Project BLAST at NCBI
|
|
| Arthropod COI sequence |
None
BLAST at The Wolbachia Project BLAST at NCBI
|
|
| Summary | The Formicidae was found to be negative for Wolbachia. | |
 European Wasp
European Wasp Small Honey Ant
Small Honey Ant 7A
7A Millipede 7F
Millipede 7F