Sample information |
|
| Picture |




|
|---|---|
| Location | |
| Collection date | 08/15/2025 |
| Captive / Cultivated? | Wild-caught |
| Group | Edmund Burke School |
| Observations | Caught indoors in a wooded area. It was sunny and 78 degrees. 6 legs, no visible wings, oval abdomen, thin antennae. 2 wings seen under microscope. They are semi-circular and sit against the abdomen. The wings are pretty small and do not extend past the end of the abdomen. It has 6 legs. The forelegs are cylindrical, with a slightly thicker upper part. The upper parts appear to have small hairs. The forelegs have several joints and are splayed outwards. The hind legs have much thicker upper parts and thinner lower parts. The lower parts of the hind legs have small spikes and the upper part has small hairs. It has a circular head with a curved face. The mouth is in the center of the face and is roughly rectangular. It has 2 medium-sized circular eyes, one on each side of the head. The eyes taper slightly towards the thorax and protrude from the head. It has 2 very long, thin antennae. Antennae are straight and extend to either side of the arthropod. It has 3 body regions. The body is roughly cylindrical with a larger abdomen that tapers slightly and a rounded face. It appears to have some sort of tail-like appendage. The top is mostly black with brown patches. The bottom is pale yellow. Legs are pale yellow with brown patches. |
| Putative identification | Arthropoda Insecta Orthoptera Gryllidae Velarifictorus Velarifictorus micado |
Methods |
|
| Extraction kit | DNeasy (Qiagen) |
| DNA extraction location | Abdomen |
| Single or Duplex PCR | Single Reaction |
| Gel electrophoresis system | MiniOne |
| Buffer | TBE |
| DNA stain | GelGreen |
| Gel images |
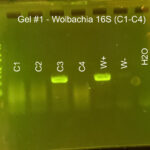
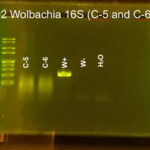
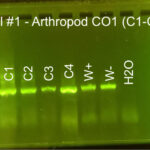
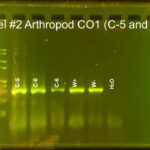
|
| Protocol notes | DNA Extraction: We added twice the listed amount of ATL and 10 extra microliters of Proteinase K. We had to remove liquid from C-3 and use an additional filter and several rounds of additional centrifuging to extract the DNA. PCR: We recombined the separate tubes from DNA extraction before starting PCR. Gel electrophoresis, Arthropod CO1: gel #1 lanes – ladder, C-1, C-2, C-3, C-4, positive Wolbachia control, negative Wolbachia control, water control; gel #2 lanes – ladder, C-5, C-6, C-6 again, Wolbachia positive control, Wolbachia negative control, water control We accidentally added C-6 DNA to two wells. All of the samples and the arthropod controls had DNA, but the water control did not. Gel electrophoresis, Wolbachia 16S: gel #1 lanes – ladder, C-1, C-2, C-3, C-4, positive Wolbachia control, negative Wolbachia control, water control; gel #2 lanes – ladder, C-5, C-6 again, Wolbachia positive control, Wolbachia negative control, water control We only added 3 microliters of W- on gel 2. Both W+ had Wolbachia DNA as did C-3. DNA sequencing: Both sequences were really good. We saved all bases with Q scores > 30. |
Results |
|
| Wolbachia presence | Yes |
| Confidence level | High |
| Explanation of confidence level | All of the samples had a band for arthropod CO1 DNA. The Wolbachia positive control had a band for both arthropod and Wolbachia DNA. The Wolbachia negative control only had a band for arthropod DNA. The water control did not have DNA in any gel. This shows that we did not have any contamination. |
| Wolbachia 16S sequence | Download FASTA
Download AB1
|
| Arthropod COI sequence | Download FASTA
Download AB1
|
| Summary | The Velarifictorus micado was found to be postive for Wolbachia. |
 Wolbachia Project
Wolbachia Project Alexander Devlin-Myrmica
Alexander Devlin-Myrmica Brookelynn Foote- Coccinellidae
Brookelynn Foote- Coccinellidae Maile Bentz- Harmonia axyridis
Maile Bentz- Harmonia axyridis Madison Adams – Harmonia axyridis
Madison Adams – Harmonia axyridis