Sample information |
|
| Picture |

|
|---|---|
| Location | |
| Collection date | 11/01/2023 |
| Captive / Cultivated? | Wild-caught |
| Group | John Overton High School |
| Observations |
|
| Putative identification | Arthropoda Insecta Coleoptera |
Methods |
|
| Extraction kit | DNeasy (Qiagen) |
| DNA extraction location | Partial abdomen |
| Single or Duplex PCR | Duplex Reaction |
| Gel electrophoresis system | Standard electrophoresis system |
| Buffer | TAE |
| DNA stain | SYBR Safe |
| Gel images |
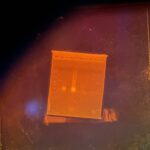
|
| Protocol notes |
DNA Purification
DNA Elution
|
Results |
|
| Wolbachia presence | No |
| Confidence level | High |
| Explanation of confidence level | There is definitely a line on my specimen that represents that it is an arthropod. Therefore, I have high confidence that the insect is negative in Wolbachia bacteria. |
| Wolbachia 16S sequence | |
| Arthropod COI sequence |
|
| Summary | The Coleoptera was found to be negative for Wolbachia. |

 European Wasp
European Wasp Small Honey Ant
Small Honey Ant 7A
7A Millipede 7F
Millipede 7F