Sample information |
|
| Picture |







|
|---|---|
| Location | |
| Collection date | 09/30/2025 |
| Captive / Cultivated? | Wild-caught |
| Group | Berkshire Community College |
| Observations | Area around the specimen was damp, but the specimen was on a pretty dry flat rock. It was collected at 9:30pm, just off the road, in a big agricultural field.
|
| Putative identification | Arthropoda Malacostraca Isopoda Armadillidae Armadillidium Armadillidium vulgare |
Methods |
|
| Extraction kit | Monarch DNA extraction (NEB) |
| DNA extraction location | Abdomen |
| Single or Duplex PCR | Duplex Reaction |
| Gel electrophoresis system | Edvotek Gel Electrophoresis |
| Buffer | 1X TAE |
| DNA stain | SYBR Safe |
| Gel images |
|
| Protocol notes | We used a DNA extraction protocol based on the insect adaptation of New England Biolabs’ Monarch Spin gDNA Extraction kit (Product # T3010). The specimen was incubated for 30 minutes in a hot water bath at 56 degrees C. Details that differed from the written protocol (incubation time differences, accidental mistakes, etc.) were:
Our first PCR reaction was set up on 10/15/2025 and was a duplex reaction because we used both the Arthropod CO1 and Wolbachia 16S primers together. We used an annealing temperature of 49 degrees Celsius. We used this Taq polymerase: New England Biolabs OneTaq Hot Start Quick-Load 2X Master Mix with Standard Buffer (#M0488S). Differed from the written protocol?
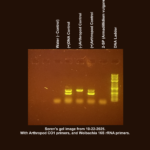
Our first Gel image was taken on 10/22/2025 and:
Our second PCR reaction was set up on 10/29/2025, used the same Taq polymerase as the first PCR reaction, and was a duplex reaction that used the Arthropod CO1_F&R and Wolbachia 16S_F&R primers.
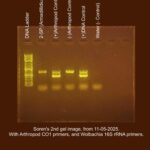
Our second Gel image was taken on 11/5/2025 and was run at 125 volts for 21 minutes.
|
Results |
|
| Wolbachia presence | No |
| Confidence level | Medium |
| Explanation of confidence level |
All the controls worked as expected. My sample (2-SP), however, showed no results, therefore I cannot determine whether my sample (2-SP) has Wolbachia, or not. This is why I have selected Unknown as my result. In the second Gel image taken on 11/5/2025 all results for the controls were as expected (same results as the first gel), but this time, my sample’s lane has a distinct band roughly 708bp, indicating that my sample (2-SP) contains arthropod DNA, but not Wolbachia DNA. Since all controls had the proper results, and the sample now shows the presence of Arthropod DNA, I am somewhat confident that my sample is negative for Wolbachia. The reason I am not highly confident is because my sample did not show any results until I increased the template DNA amount and ran another duplex PCR. The level of Wolbachia, if present, could still be too little to detect. |
| Wolbachia 16S sequence | |
| Arthropod COI sequence | Download AB1
GAGCAGGGGCTGTTGGGACTGCTCTTAGAATAATTATTCGCACTGAATTAGGACAACCTGGAAGTTTAATTGGAGATGATCAGATTTATAATGTAATTGTAACTGCTCATGCTTTTGTAATAATTTTTTTTATAGTAATACCTATTATAATTGGTGGATTCGGTAATTGGTTAATTCCATTAATACTAGGAGCCCCAGATATAGCTTTTCCACGAATAAATAATATAAGATTTTGACTCTTACCCCCTTCTTTAACTTTATTACTGAGAAGGGGATTAGTTGAAAGAGGAGTAGGAACAGGGTGAACAGTGTATCCTCCGCTGGCGTCAAGAATTGCTCATAGAGGAGCATCTGTAGATTTAGGTATTTTTTCTCTCCACCTTGCTGGAGCCTCTTCTATTTTAGGGGCTGTAAATTTTATTACTACTATAATTAATATACGAGCAGCTGGAATTAGAATAGACCGTGTTCCTTTATTTGTTTGATCAGTAATAGTAACGGCTGTACTTTTACTTTTATCATTACCTGTATTAGCAGGAGCTATTACTATATTATTGACTGATCGAAATTTAAATACATCTTTTTTTGACCCTAGAGGAGGGGGGGATCCTGTCCTTTATCAACATTTATTTTGA
BLAST at The Wolbachia Project BLAST at NCBI
|
| Summary | The Armadillidium vulgare was found to be negative for Wolbachia. |
 Wolbachia Project
Wolbachia Project Alexander Devlin-Myrmica
Alexander Devlin-Myrmica Brookelynn Foote- Coccinellidae
Brookelynn Foote- Coccinellidae Maile Bentz- Harmonia axyridis
Maile Bentz- Harmonia axyridis Madison Adams – Harmonia axyridis
Madison Adams – Harmonia axyridis